3. 结构
3.1 二维结构
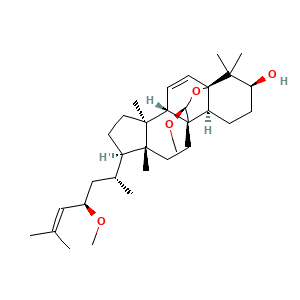
3.2 三维结构
-1
-2
-3
88 92 0 1 0 0 0 0 0999 V2000
4.3012 0.5021 -0.6323 O 0 0 0 0 0 0 0 0 0 0 0 0
3.7248 2.4319 0.5708 O 0 0 0 0 0 0 0 0 0 0 0 0
5.9177 -0.3304 1.6067 O 0 0 0 0 0 0 0 0 0 0 0 0
-5.6334 0.7240 1.8600 O 0 0 0 0 0 0 0 0 0 0 0 0
1.9503 0.8071 -0.0760 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.3037 0.0702 -1.2346 C 0 0 1 0 0 0 0 0 0 0 0 0
1.2014 0.4270 -1.4378 C 0 0 2 0 0 0 0 0 0 0 0 0
-1.0237 1.2543 -0.4981 C 0 0 1 0 0 0 0 0 0 0 0 0
2.5413 -0.4843 0.5659 C 0 0 1 0 0 0 0 0 0 0 0 0
3.6798 -0.7895 -0.4405 C 0 0 2 0 0 0 0 0 0 0 0 0
1.0733 1.7000 0.8536 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.5019 0.8008 -0.5873 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.4330 1.3891 0.9111 C 0 0 0 0 0 0 0 0 0 0 0 0
3.3009 1.5288 -0.4441 C 0 0 2 0 0 0 0 0 0 0 0 0
-1.1631 -0.0270 -2.5146 C 0 0 0 0 0 0 0 0 0 0 0 0
4.7235 -1.8488 0.0698 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.6098 0.2495 -2.0343 C 0 0 0 0 0 0 0 0 0 0 0 0
3.0137 -0.3086 2.0225 C 0 0 0 0 0 0 0 0 0 0 0 0
1.9550 -0.6063 -2.2422 C 0 0 0 0 0 0 0 0 0 0 0 0
3.0989 -1.1473 -1.8028 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.4629 -1.3158 -0.5251 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.8882 2.6409 -1.2116 C 0 0 0 0 0 0 0 0 0 0 0 0
5.1616 -1.5409 1.5323 C 0 0 2 0 0 0 0 0 0 0 0 0
-3.5707 1.8414 -0.2744 C 0 0 1 0 0 0 0 0 0 0 0 0
3.9746 -1.3959 2.4816 C 0 0 0 0 0 0 0 0 0 0 0 0
5.9862 -1.8674 -0.8293 C 0 0 0 0 0 0 0 0 0 0 0 0
4.1177 -3.2779 0.0437 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.9993 1.2720 -0.3950 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.3576 2.3953 1.1390 C 0 0 0 0 0 0 0 0 0 0 0 0
4.9434 3.0724 0.2209 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.3446 0.1394 0.5866 C 0 0 2 0 0 0 0 0 0 0 0 0
-6.5691 -0.6272 0.1692 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.6964 -1.9529 -0.0316 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.4834 -0.2229 2.9045 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.6067 -2.9725 0.1408 C 0 0 0 0 0 0 0 0 0 0 0 0
-8.0176 -2.5307 -0.4672 C 0 0 0 0 0 0 0 0 0 0 0 0
1.2331 1.3183 -2.0773 H 0 0 0 0 0 0 0 0 0 0 0 0
1.8378 -1.3172 0.5761 H 0 0 0 0 0 0 0 0 0 0 0 0
1.1772 2.7419 0.5228 H 0 0 0 0 0 0 0 0 0 0 0 0
1.4561 1.7095 1.8790 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.6229 -0.0330 0.1110 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.6037 0.4918 1.5131 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.8779 2.2055 1.4794 H 0 0 0 0 0 0 0 0 0 0 0 0
3.1993 2.0897 -1.3811 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.8647 0.7246 -3.2547 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.0907 -1.0024 -3.0087 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.0892 0.9584 -2.7188 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.2052 -0.6705 -2.0460 H 0 0 0 0 0 0 0 0 0 0 0 0
2.1405 -0.3126 2.6866 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5047 0.6561 2.1758 H 0 0 0 0 0 0 0 0 0 0 0 0
1.5832 -0.8708 -3.2274 H 0 0 0 0 0 0 0 0 0 0 0 0
3.6281 -1.8294 -2.4596 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.2044 -1.2904 0.5335 H 0 0 0 0 0 0 0 0 0 0 0 0
0.1560 -2.0817 -1.0045 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.4846 -1.7077 -0.5760 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.3890 2.6674 -2.1844 H 0 0 0 0 0 0 0 0 0 0 0 0
0.1480 2.9345 -1.3936 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.3196 3.4471 -0.6106 H 0 0 0 0 0 0 0 0 0 0 0 0
5.8170 -2.3411 1.8979 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.5151 2.6770 -0.9827 H 0 0 0 0 0 0 0 0 0 0 0 0
4.3312 -1.1381 3.4866 H 0 0 0 0 0 0 0 0 0 0 0 0
3.4387 -2.3465 2.5761 H 0 0 0 0 0 0 0 0 0 0 0 0
6.7324 -2.5665 -0.4335 H 0 0 0 0 0 0 0 0 0 0 0 0
6.4608 -0.8822 -0.8873 H 0 0 0 0 0 0 0 0 0 0 0 0
5.7607 -2.1860 -1.8517 H 0 0 0 0 0 0 0 0 0 0 0 0
4.7902 -3.9968 0.5264 H 0 0 0 0 0 0 0 0 0 0 0 0
3.1535 -3.3294 0.5581 H 0 0 0 0 0 0 0 0 0 0 0 0
3.9591 -3.6348 -0.9796 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.1481 0.9202 -1.4236 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.7093 2.0991 -0.2535 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.1003 1.6195 1.8664 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.2650 2.8969 1.4965 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.5985 3.1795 1.1526 H 0 0 0 0 0 0 0 0 0 0 0 0
6.2089 -0.2198 2.5278 H 0 0 0 0 0 0 0 0 0 0 0 0
5.7677 2.3544 0.1781 H 0 0 0 0 0 0 0 0 0 0 0 0
5.1749 3.8077 0.9965 H 0 0 0 0 0 0 0 0 0 0 0 0
4.8478 3.5983 -0.7341 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.4897 -0.5280 0.6824 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.4520 -0.0064 0.0151 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.6620 0.2911 3.8534 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.4656 -0.6256 2.9258 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.2112 -1.0351 2.8221 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.4166 -3.4818 -0.8099 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.9106 -3.7238 0.8776 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.6576 -2.5552 0.4813 H 0 0 0 0 0 0 0 0 0 0 0 0
-8.7905 -1.7643 -0.5874 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.9079 -3.0441 -1.4282 H 0 0 0 0 0 0 0 0 0 0 0 0
-8.3783 -3.2518 0.2736 H 0 0 0 0 0 0 0 0 0 0 0 0
1 10 1 0 0 0 0
1 14 1 0 0 0 0
2 14 1 0 0 0 0
2 30 1 0 0 0 0
3 23 1 0 0 0 0
3 74 1 0 0 0 0
4 31 1 0 0 0 0
4 34 1 0 0 0 0
5 7 1 0 0 0 0
5 9 1 0 0 0 0
5 11 1 0 0 0 0
5 14 1 0 0 0 0
6 7 1 0 0 0 0
6 8 1 0 0 0 0
6 15 1 0 0 0 0
6 21 1 0 0 0 0
7 19 1 0 0 0 0
7 37 1 0 0 0 0
8 12 1 0 0 0 0
8 13 1 0 0 0 0
8 22 1 0 0 0 0
9 10 1 0 0 0 0
9 18 1 0 0 0 0
9 38 1 0 0 0 0
10 16 1 0 0 0 0
10 20 1 0 0 0 0
11 13 1 0 0 0 0
11 39 1 0 0 0 0
11 40 1 0 0 0 0
12 17 1 0 0 0 0
12 24 1 0 0 0 0
12 41 1 0 0 0 0
13 42 1 0 0 0 0
13 43 1 0 0 0 0
14 44 1 0 0 0 0
15 17 1 0 0 0 0
15 45 1 0 0 0 0
15 46 1 0 0 0 0
16 23 1 0 0 0 0
16 26 1 0 0 0 0
16 27 1 0 0 0 0
17 47 1 0 0 0 0
17 48 1 0 0 0 0
18 25 1 0 0 0 0
18 49 1 0 0 0 0
18 50 1 0 0 0 0
19 20 2 0 0 0 0
19 51 1 0 0 0 0
20 52 1 0 0 0 0
21 53 1 0 0 0 0
21 54 1 0 0 0 0
21 55 1 0 0 0 0
22 56 1 0 0 0 0
22 57 1 0 0 0 0
22 58 1 0 0 0 0
23 25 1 0 0 0 0
23 59 1 0 0 0 0
24 28 1 0 0 0 0
24 29 1 0 0 0 0
24 60 1 0 0 0 0
25 61 1 0 0 0 0
25 62 1 0 0 0 0
26 63 1 0 0 0 0
26 64 1 0 0 0 0
26 65 1 0 0 0 0
27 66 1 0 0 0 0
27 67 1 0 0 0 0
27 68 1 0 0 0 0
28 31 1 0 0 0 0
28 69 1 0 0 0 0
28 70 1 0 0 0 0
29 71 1 0 0 0 0
29 72 1 0 0 0 0
29 73 1 0 0 0 0
30 75 1 0 0 0 0
30 76 1 0 0 0 0
30 77 1 0 0 0 0
31 32 1 0 0 0 0
31 78 1 0 0 0 0
32 33 2 0 0 0 0
32 79 1 0 0 0 0
33 35 1 0 0 0 0
33 36 1 0 0 0 0
34 80 1 0 0 0 0
34 81 1 0 0 0 0
34 82 1 0 0 0 0
35 83 1 0 0 0 0
35 84 1 0 0 0 0
35 85 1 0 0 0 0
36 86 1 0 0 0 0
36 87 1 0 0 0 0
36 88 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
(1R,4S,5S,8R,9R,12S,13S,16S,19S)-19-methoxy-8-[(2R,4R)-4-methoxy-6-methylhept-5-en-2-yl]-5,9,17,17-tetramethyl-18-oxapentacyclo[10.5.2.01,13.04,12.05,9]nonadec-2-en-16-ol
4.2 InChl
InChI=1S/C32H52O4/c1-20(2)18-22(34-8)19-21(3)23-12-14-30(7)24-13-15-32-25(10-11-26(33)28(32,4)5)31(24,27(35-9)36-32)17-16-29(23,30)6/h13,15,18,21-27,33H,10-12,14,16-17,19H2,1-9H3/t21-,22+,23-,24+,25+,26+,27+,29-,30+,31+,32-/m1/s1
4.3 InChlKey
CRZHMFVUXYIFSA-HRNSCMLOSA-N
4.4 Canonical SMILES
CC(CC(C=C(C)C)OC)C1CCC2(C1(CCC34C2C=CC5(C3CCC(C5(C)C)O)OC4OC)C)C
4.5 lsomeric SMILES
C[C@H](C[C@H](C=C(C)C)OC)[C@H]1CC[C@@]2([C@@]1(CC[C@]34[C@H]2C=C[C@]5([C@H]3CC[C@@H](C5(C)C)O)O[C@@H]4OC)C)C
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病